Soil testing sometimes reveals adequate nutrient levels, yet plants are not growing and developing normally. This may be due to an excess of free hydrogen ions (H+) formed during chemical reactions in the soil, leading to increased acidity.
What is acidity and its types?
- ✓ pH levels should be measured at several points across the site to obtain representative data.
- ✓ For accurate soil pH measurements, it is recommended to use distilled water rather than tap water to avoid distortion due to minerals.
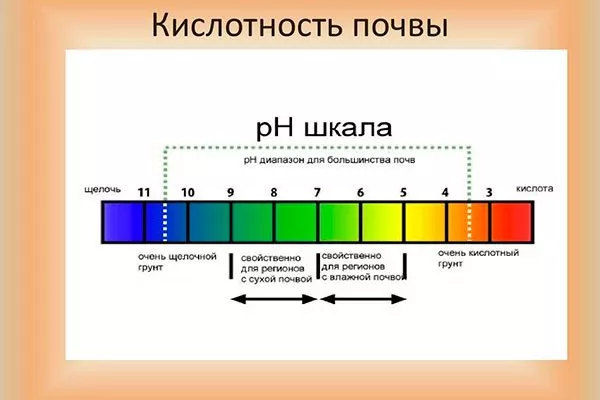
Acidity is a characteristic of a medium that reflects the activity of positive hydrogen ions within it. The pH index is a measure of this activity and comes from the Latin phrase "pondus hydrogenii," meaning "weight of hydrogen." High H+ ion activity indicates an acidic substrate and a correspondingly lower pH.
Soil acidity, designated by the pH index, depends on the quantity and ratio of chemical elements. Experiments show that plants, including vegetable and berry crops, absorb nutrients best at a pH between 6.0 and 7.0. Soil with a pH of 7.0 is considered neutral.
Any pH value below 7.0 indicates soil acidity, with the lower the number, the higher the acidity. Below is a table showing different soil types by their acidity:
| Soil acidity level meaning | Units in pH | Substrate type |
| Highly acidic | from 0 to 4.5 | peat lowland, marsh |
| Sour | from 4.5 to 5.3 | coniferous, sod-clay, peat |
| Subacid | from 5.3 to 6.3 | turf, heather |
| Neutral | from 6.3 to 7.3 | foliar |
| Slightly alkaline | from 7.3 to 8.0 | humus |
| Alkaline | from 8.0 to 8.5 | carbonate |
| highly alkaline | from 8.5 to 9.0 and above | carbonate |
How does soil acidity affect plant life?
Many vegetable and berry crops are unable to grow and develop normally in acidic soils because such conditions produce compounds that plant roots are unable to absorb.
Although nutrients are present in the soil, plants experience a deficiency due to their inaccessibility, which leads to the cessation of their growth and development.
Other negative factors:
- Excessive soil acidity reduces soil fertility and negatively impacts plant life.
- Organic acids in high concentrations disrupt protein metabolism in cells, slow down the growth of the root system and can lead to its death.
- The availability of essential elements such as phosphorus, potassium, calcium and magnesium is reduced, while aluminum, boron, iron and zinc can reach concentrations that are toxic to plants.
- It reduces the activity of beneficial microorganisms that enrich the fertile soil layers with nitrogen, and promotes the development of pathogenic fungi, bacteria, and viruses.
- It interferes with the movement of phosphorus to the above-ground parts of the plant, causing a deficiency of this element.
- Leads to a weakening of the processes of processing organic matter into humus and subsequent transformation into forms accessible for assimilation by plants.
An excessively alkaline environment (pH > 7.5–8) also has a negative impact on plant health, as many microelements important for their growth are converted into insoluble hydroxides and become unavailable for nutrition.
Other negative impacts:
- An excess of alkali metal salts, such as sodium carbonate, is detected, leading to salinity. Due to the swelling properties of these salts, the soil's permeability to water is impaired, leading to moisture stagnation and the formation of a surface crust that impedes air access to plant roots.
- The nutritional value of alkaline soils is low because vital elements such as phosphorus, iron, zinc and molybdenum are in forms that are difficult for plants to absorb.
- Poor aeration of the root system further aggravates the situation, preventing plants from functioning normally and developing fully.
What soil acidity is best for which plants?
Most cultivated plants prefer neutral soil pH, but some species can adapt to slightly altered pH levels—usually slightly acidic. For garden and vegetable plants, it's important to maintain an optimal soil pH, which typically falls within the following pH ranges:
- for watermelon, potatoes, pumpkin, parsnips and sorrel – pH 5.0–6.0;
- for vegetable crops such as tomatoes, cabbage, carrots, corn, garlic, peppers, cucumbers, beets and peas – pH 5.5–7.0;
- for leafy salads, onions, legumes and other vegetable crops – pH 6.0–7.0;
- for cauliflower, artichoke, celery, asparagus and parsley - pH 7.0–7.8.
- ✓ For most vegetable crops, the optimal soil pH should be in the range of 6.0-7.0, which ensures the best availability of nutrients.
- ✓ Some plants, such as blueberries and rhododendrons, require more acidic soil with a pH of 4.5-5.5.
Ornamental and forest plants also have their own preferences in terms of acidity:
- plants that prefer acidic soils, such as heather, hydrangea and erica – pH 4.0–5.0;
- fruit trees like plum and cherry – pH approximately 6.0–7.0;
- For apple, pear and strawberry trees, the optimal pH range is this is 5.5–7.0.
It's worth noting that some plants don't tolerate excessively acidic soil, such as asparagus, most cabbages and peppers, celery, beets, and clematis. Roses, strawberries, pears, apples, and clover can suffer from high soil salt levels.
Why and how to determine soil acidity?
There are various methods to determine the acidity level of the soil; they are conventionally divided into precise and approximate.
Inaccurate
As the name suggests, some methods can only provide a general idea of the soil's character, indicating whether it is acidic, neutral, or alkaline. These methods include:
- folk methods (vinegar, etc.);
- using crushed chalk;
- litmus paper testing;
- monitoring the reaction of indicator plants.
Accurate
However, there are more informative measurement methods that allow one to determine the exact numerical value of soil acidity, or pH level. These methods include:
- laboratory analysis, which has the advantage of accuracy of the result, but has disadvantages such as time and financial costs;
- using a pH meter, which guarantees accurate results, is easy to operate and provides instant measurements, but requires an initial investment to purchase.
How to determine soil acidity?
Every gardener can use any of the existing methods for determining soil acidity, but in this case it is important to strictly follow all recommendations.
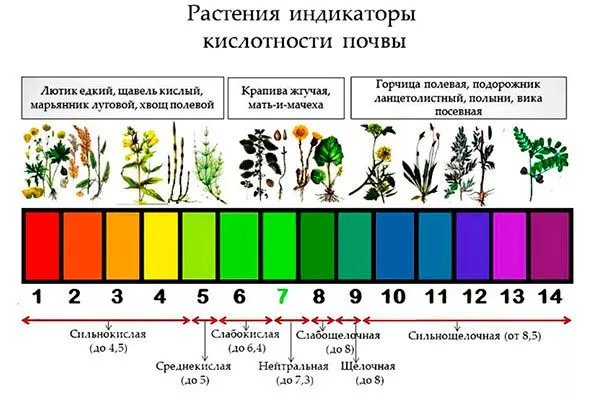
Indicator plants
To independently determine the acidity of the soil, many advise paying attention to the wild herbs growing in a given area:
- in acidic clearings horse sorrel, various types of plantain, field horsetail, common mint, wood sorrel, fireweed, heather, wild mustard, blue lupine, creeping buttercup and the like are found;
- on alkaline soils larkspur, wild poppy, field mustard, beans, and stachys grow more often;
- on neutral or slightly acidic soilSuitable for most agricultural crops, you can find coltsfoot, field bindweed, various types of clover, wild radish, clover, thistle, nettle, eryngium, etc.
Acidity measuring devices
For these measurements, specialized devices called pH meters exist. They are available in two main types: analog and digital. They operate by measuring the electromotive force, which correlates with the activity of hydrogen ions. The device's scale is calibrated in pH units, making it easier to interpret the measurements.
For home measurements, you can use portable analyzers such as pH meters, acid meters, and soil probes. These devices are easy to operate: simply insert the probe into the soil, and after a short time, the device's display will show the acidity level.
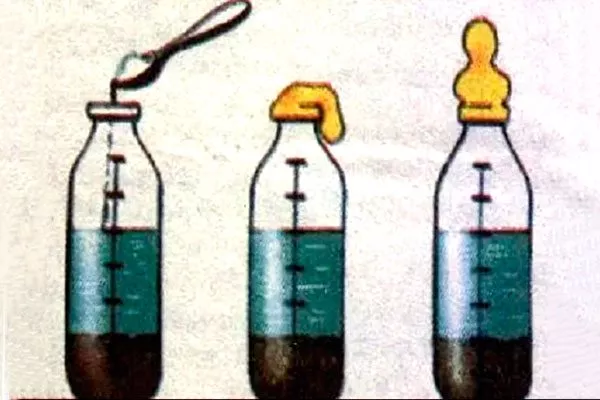
Indicator strips
Litmus strips are another way to assess acidity levels. To do this, perform the following analysis:
- On the site, dig holes with straight, smooth sides to a depth equal to the depth of a shovel blade.
- Carefully remove a thin layer of soil from one of the vertical sides of the hole, mix it on a clean surface, for example, on a film, and take a sample weighing about 15-20 grams.
- Then you need to mix the soil in clean water, wait until it clears up and immerse an indicator paper strip in the water.
The color range varies and changes depending on the acidity level:
- when the strip turns red, this indicates an acidic soil reaction;
- orange – about medium acid reaction;
- yellow color - about slightly acidic reaction;
- light green – about neutral reaction;
- shades of blue – about the alkaline reaction of the soil.
How to determine acidity at home?
There are "grandmother's" methods that are also inaccurate, but users report quite acceptable results. However, this is debatable.
Baking soda and vinegar
First, prepare an aqueous soil extract: thoroughly grind 200 g of soil, place it in a container, and add 1 liter of distilled water, previously boiled to remove dissolved gases. This solution should be thoroughly mixed for at least 5 minutes and then left to settle for a while.
The vinegar and baking soda test involves the following:
- Soda and vinegar are added to two different samples of the aqueous extract.
- If a gas-releasing reaction is observed in the vinegar sample, the soil is alkaline.
- If the sample reacts with soda, the soil is acidic.
Grape juice
You can use grape juice (avoid wine). Drop a lump of soil into a glass of juice and observe the color change and the formation of bubbles, which indicates neutral soil pH.
Currant or cherry leaves
The leaves are used as follows:
- pour boiling water over them;
- leave for 15-20 minutes;
- add a lump of earth.
By appearance
The following signs indicate increased acidity:
- Grayish coating, gray soil color or presence of podzol under the turf layer.
- Characteristic plants include plantain, horsetail, chickweed, buttercup and sorrel.
- After rain, the water in puddles has a rusty color and there are whitish spots resembling ash under the fertile soil layer.
How to increase acidity levels?
Various technologies are used for this purpose. Each has its own characteristics that must be ignored for the procedure to be successful.
Sulfur
For sulfur to be effectively utilized as a chemical element, moisture is essential. When reacting with water, sulfur converts to sulfuric acid, which lowers the pH. The oxidation process is lengthy and can take up to a year. However, it can be accelerated by using finely ground sulfur, applied at a rate of 110-140 g per square meter, which is sufficient to lower the pH by 2.5 points.
When using sulfur, it's crucial to consider weather conditions because it's easily carried by the wind. Colloidal sulfur can be used, applied a year before planting at a dose of 4-5 g per 10 liters of soil mixture.
Aluminum sulfate
To reduce the pH by one unit, apply 100 g of the substance per 1.5 square meters. This method works faster than sulfur, with results occurring within 2.5 weeks. Excessive use of aluminum sulfate can reduce the availability of phosphorus in the soil, so it is advisable to apply phosphate fertilizers after applying it.
It is important to remember the potential toxicity of aluminum, which can accumulate in vegetables and have harmful effects on the human body. Therefore, its use is recommended in strictly specified doses and not every year.
Ferrous sulfate
This chemical can acidify soil similarly to aluminum sulfate, while enriching it with iron, which is essential for plant development. The recommended dosage is 90-100 g per square meter, with an expected pH reduction effect within a month. As with aluminum sulfate, due to reduced availability of phosphorus, it is beneficial to apply phosphorus-containing fertilizers after soil acidification.
Potassium sulfate
This type of fertilizer is typically applied in the fall. Potassium sulfate is a gentle acidity corrector, suitable for soils where a slightly acidic environment is preferred. The recommended rate is up to 50 g per square meter.
Ammonium nitrate
This fertilizer produces a slight lowering effect and can be used in conjunction with other pH regulation methods. It should be applied in the spring before soil cultivation.
Sowing green manure
Using green manure is one of the easiest and most environmentally friendly methods. Suitable green manures include white mustard, oats, rapeseed, and rapeseed. They are sown in early spring and, once the green mass has formed, mowed, and then left to grow directly on the plot.
Electrolytes of acid batteries
To regulate soil acidity, you can also use an electrolyte containing sulfuric acid (from lead-acid batteries). It should be applied diluted at a ratio of 50 ml per 10 liters of water. The prepared solution is used to treat 1 square meter of land.
Vinegar and citric acid
Citric acid and vinegar are common kitchen items. However, it's worth noting that the effects of these remedies are temporary and mild. Vinegar should only be used when necessary, as it can negatively impact beneficial microorganisms in the soil.
It is recommended to dilute vinegar (9%) at a ratio of 100 ml per 10 liters of water before watering. Citric acid, which is a gentler solution, is added at a ratio of 1.5 teaspoons per 10 liters of water.
Coffee grounds
Coffee lovers can use leftover coffee grounds as a fertilizer and soil acidifier. Coffee grounds contain nitrogen, potassium, and phosphorus, which are valuable plant nutrients. They can be applied alone or mixed with conifer bark or pine needles, incorporating them into the soil in the fall before tilling.
Other methods
There are other options:
- Add high-moor red peat to the soil when digging – approximately 1.5-2.5 kg per 1 sq. m, which will improve the soil structure and increase its acidity.
- Use fresh manure or cow dung – up to 2.5 kg per 1 sq. m.
- Mulching the soil with semi-rotted pine needles or sawdust – in the amount of 3-4.5 kg per 1 sq. m.
How to deacidify soil on a plot?
Before attempting to reduce soil acidity in your garden, you need to plan the area. It's important to determine which areas require soil testing. Then, conduct a soil analysis and adjust the soil acidity levels if necessary.
Liming
Liming is the most common method for reducing acidity, using materials such as slaked lime, dolomite flour, chalk, or lake lime. Lime application rates depend on the soil type and its degree of acidity.
Traditionally, liming is carried out:
- for heavy soils - every 5-7 years;
- for lungs - every 4-5 years;
- for peat - every 3 years.
Typically, it affects a soil layer up to 20 cm deep. If lime is applied in smaller quantities, only the top 6-8 cm deep layer is treated. After spreading the lime over the surface of the beds, watering is recommended. The soil will reach a neutral pH and decrease in acidity after a couple of years.
Liming should not be combined with fertilization; these processes should be separated: deoxidation should be carried out in the fall, and fertilization should be carried out in the spring. Otherwise, this can lead to the formation of compounds that limit the availability of nutrients to plants.
Recommended doses of fluff per 1 sq.m:
- for acidic soils – 500 g;
- for soils with average acidity – 300 g;
- for slightly acidic soils – 200 g.
Before starting work, measure the required amount of reagent. Then, spread it evenly over the soil surface and bury it to the depth of a spade. This will normalize the acidity of the soil layer to a depth of 15 to 20 cm.
Ash
Wood ash has the ability to neutralize excess soil acidity. It also repels pests and serves as a good fertilizer. However, there are a few important points to keep in mind when using it:
- The composition of ash can vary greatly depending on the type and age of wood burned, where it grows, and other factors.
- The calcium content of ash can range from 30% to 60%, which affects the recommended application rates. On average, 1 to 1.5 kg per square meter can be added.
- Birch ash is especially useful, as it contains additional nutrients such as phosphorus and potassium.
- It's not recommended to use ash from burning weeds and tops, as it lacks calcium. The application rate for this type of ash is 2-2.5 kg per square meter, and obtaining this amount can be difficult. It's usually added as a supplement to other fertilizers or used a year after the main liming.
To prepare the solution, dissolve 200 g of charcoal in 1 liter of water, which is enough to treat 1 square meter of soil. If using peat ash, increase the dosage to 250-300 g.
Dolomite flour
Dolomite flour is gentler than lime and contains calcium and magnesium carbonate, which helps improve fertility. Dolomite is a finely ground mineral similar to limestone that regulates acidity and provides micro- and macronutrients. It is excellent for loosening heavy soils and improving their structure.
The product is available in gardening and hardware stores in various packaging sizes. For best results, it is recommended to choose the finest dolomite grain size, no larger than 0.25 mm, with a moisture content of no more than 15%, as indicated on the packaging.
Dolomite flour is a mild fertilizer and can be applied during spring or fall tillage. The recommended amount depends on the soil's acidity:
- for soured - 0.5 kg;
- for soil with medium acidity – 0.4 kg;
- for slightly acidic soils – 0.3 kg.
Additionally, it helps combat fungal diseases and certain types of pests by destroying the chitinous covering of insects such as mole crickets and Colorado potato beetles.
Chalk and plaster
Chalk is used similarly to lime fertilizers: it is ground to a particle size of no more than 1 mm in diameter to ensure rapid dissolution and activation in the soil. If the particle size is larger, the chalk's effect on the soil will be delayed.
To deoxidize, the finely ground material should be evenly distributed over the area, and then added to the soil during digging, observing certain standards for 1 square meter:
- Acidified soil: 500-700 g.
- Medium acid soil: 400 g.
- Slightly acidic soil: 250-300 g.
Gypsum has a composition similar to chalk, but its action is more selective, as it reacts only with acids in the soil. Once applied, it neutralizes the acid and becomes inactive until the next change in the pH balance. Gypsum does not harm soil microorganisms or plants. Gypsum application rates:
- Acidified substrates: 350-450 g.
- Medium acidity: 250-350 g.
- Subacid: 150-250 g.
The effect of both chalk and gypsum is short-lived, so regular adjustments to the substrate composition are required. Continuous use is not recommended, as they can accumulate in the soil and lead to salinization.
Green manure
Agronomists recommend using green manure crops—plants that deacidify the soil while enriching it with nutrients. Suitable green manure crops include lupine, rapeseed, mustard, phacelia, oats, sweet clover, oilseed radish, wheat, and others. Sowing is done in early spring, as these crops are resistant to spring frosts.
Soil acidity instability
You can't rely on a single acidity measurement to base a long-term action plan on. Soil acidity can vary significantly over time under the influence of various factors, including precipitation, irrigation, groundwater, fertilizers, and even plant root activity.
Problems of acidic soils
If acidity is elevated, nitrogen mineralization does not occur because the activity of important bacteria is suppressed, causing nitrogen deficiency. Furthermore, this negatively impacts the population of beneficial microorganisms and bacteria, leading to decreased production of essential nutrients required for healthy plant growth.
If soil contains high concentrations of heavy metals such as aluminum, toxic compounds can form and penetrate the root system of plants, causing damage and impairing their ability to absorb nutrients.
Problems of soils with high alkaline levels
Alkaline soils are characterized by elevated levels of alkaline elements such as calcium (Ca), magnesium (Mg) and sodium (Na), which contribute to soil salinization and reduce the availability of important micronutrients including iron (Fe), phosphorus (P), zinc (Zn) and molybdenum (Mo).
Such soils usually have a poor structure and after rain the top layer tends to form a crust, while the bottom layer does not allow water to pass through well.
To maintain the desired pH level of the soil mixture, it is necessary to regularly improve it. Adjustments are one of the effective methods for increasing crop yields. However, such changes affect the soil ecosystem and should be made carefully, following the recommendations for the use of specialized fertilizers and products at precise dosages.